Structure of Matter and Molecule
What is Matter
Different objects present around us, like- water, air, salt, book, computer etc., are all matter. Every object that occupies space has mass and which can be sensed with the five sense organs, is termed as matter.
When we say that matter has mass, it means that it has weight. The more heavy an object is, greater will be its mass. Matter occupies space means that it has volume.
- Properties of Matter :
- 1. There is empty space between the particles of matter.
- 2. The particles of matter are in a state of constant motion.
- 3. The particles of matter attract each other.
- Types of Matter : Matter can be divided into two types on the basis of its components :
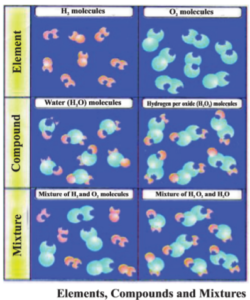
- 1. Pure Matter : Matter which has only one type of ingredient or component is known as pure matter. For example : Iron, Gold, Water, Oxygen, etc. Elements and components are pure matter.
- 2. Impure Matter : Matter which has more than one type of ingredients or components are known as impure matter. For example : Cold drink, soil, air etc. Mixtures are impure matter.
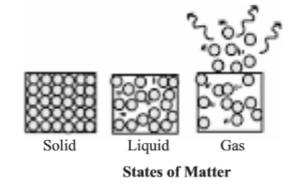
- States of Matter : On the basis of physical state, matter can be classified into three phases :
- (i) Solid
- (ii) Liquid
- (iii) Gas
- For example :
- H2O(g) gaseous phase – steam
- H2O(l) liquid phase – water
- H2O(s) solid phase – Ice
- Now scientists are considering five states of matter by including
- For example :
- (iv) Plasma
- (v) Bose – Einstein Condensate (BEC)
- Characteristic Properties of Matter: The three basic states of matter can be identified on the basis of their specific properties.
- 1. Solid State: There are numerous substances in solid form all around us. For example : Piece of wood, stone, pencil, pen, computer, salt etc. Following are the characteristic properties of the solid state :
- (i) Solid has a definite shape
- (ii) Solid has a definite volume
- (iii) The density of solid is more
- (iv) Compressibility of solid is negligible
- (v)A high inter-molecular force of attraction is present in between the particles of solid state.
- (vi) Diffusion in particles of solid is extremely less.
- 2. Liquid State : Water, mustard oil, kerosene etc. are the examples of liquid. The volume of liquid is definite but its shape is not; they take the shape according to the vessel. Liquid can flow. Liquid can be poured or spread. The properties of liquid are intermediate between solid and gas. The characteristic properties of liquid state are :
- (i) Shape of liquid is not definite.
- (ii) Volume of liquid is definite.
- (iii) The density of liquid is more than that of gas but less than that of solid.
- (iv) The compressibility of liquid is very less.
- (v) The inter-molecular force of attraction between the particles of liquid is weak.
- (vi) Diffusion in particles of liquid is less than that in gas but more than that in solid.
- 3. Gas State : The air present around us is the best example of the gas state, other examples include – Oxygen, Nitrogen, Argon, Carbon-di-oxide etc. Following are the characteristic properties of the Gas state :
- (i) The shape of gas is not definite and ittakes the shape of the vessel, in which it is placed.
- (ii) The volume of gas is not definite and it take its volume according to the shape of the vessel.
- (iii) The density of gas is very less.
- (iv) The compressibility of gas is very high.
- (v) The inter-molecular force of attraction between the particles of gas is negligible.
- (vi) The diffusion in the particles of gas is very high. Hence it quickly spreads every where.
- 1. Solid State: There are numerous substances in solid form all around us. For example : Piece of wood, stone, pencil, pen, computer, salt etc. Following are the characteristic properties of the solid state :
The distance between the particles of gas is too much. They can be brought near each other by applying high pressure and reducing the temperature and can be liquefied. The name of CNG, used as a fuel, is Compressed Natural Gas. LPG is Liquefied Petroleum Gas.
What is Kanad Theory
Ancient Bhartiya and Greek philosophers have always been astonished by the unknown and invisible forms of matter. Ideas had been expressed in Bharat about the concept of indivisibility of matter, as early as, in about 500 BC.
Bhartiya philosopher Maharshi Kanad had postulated that if we go on dividing matter, we will be obtaining smaller particles and in the end we will reach the limit when the particle obtained cannot be further divided i.e. that minutest particle will be indvisible. He named this indivisible, minute particle as the ‘parmanu’. Another Bhartiya philosopher Pakudha Katyayana explained this idea in an elaborate form and stated that these particles are usually found in linked forms, which provide us with the different types of matter (element, compound, mixture).
Approximately at the same time i.e. 460 to 370 BC, Greek philosopher Democritus and Lencippus, suggested that if we keep on dividing matter a point will be reached when the particle obtained cannot be divided further. They termed these indivisible particles as atoms (i.e. uncuttables).
All the above mentioned views were on the basis of philosophical ideas. In 1808 John Dalton proposed the Atomic Theory and the credit of discovering atom was given to him.
Dalton suggested the view of divisibility of matter which was then considered philosophical. The minutest, indivisible particles of matter, which were termed atom by the Greek philosophers, were designated by Dalton also, as atom. This theory of Dalton was based on the laws of chemical combination. Dalton’s Atomic Theory provided rational analysis of the law of Conservation of Mass and Law of Definite Proportions.
What is Atom ?
According to Dalton’s Atomic Theory, all matter, whether element, compound or mixture, are made up of minute particles called atom. Atoms are the minutest particles. Their shape is approximately –10of the range of 10 m. The atoms of most of the elements cannot exist independently. Atoms form molecules and ions. These molecules or ions group in large numbers to form matter, which can be seen, felt or touched by us.
What is Molecule?
Generally molecule is a group of two or more atoms which are joined together by chemical bonds, which cannot be separated by general physical methods. Therefore, the minutest particle of an element or compound which can exist independently and expresses all the characteristic properties of that compound, is termed as a molecule. For example : molecules of salt, molecules of phosphorus etc.
What is Elements ?
Group of same type of atoms is known as Element. For example : Gold, silver, iron, sulphur etc. Till date 118 elements are known. The molecule of an element is made up of a combination of one or more than one atoms. For example : Molecules of Argon, Helium etc. are made up of a single atom of the element while the two types of molecules of oxygen are O2 and O3 which are made up of two and three atoms of oxygen, respectively. O2 is known as di-oxygen and O3 is known as Ozone. The number of atoms of an element present in a molecule is known as the atomicity of that element. The atomicity of oxygen in ozone is 3.
What is Compound ?
The substance formed by the chemical combination of 2 or more than two elements in a definite ratio is termed as a Compound. For example: Salt, Water, Ammonia, Sulphuric acid etc. The smallest particle of a compound which can exist independently and possess all the properties of the compound is known as a molecule of the compound. For example : Water : molecular formula H2O, Ammonia : molecular formula NH3 etc.
What is Mixture ?
The substance formed by mixing two or more than two elements or compounds in uncertain quantity, is known as a mixture. There is no chemical bonding between its components. Hence they can be separated by simple physical methods for example : air is a mixture, whose components includes N2, O2, CO2, H2O etc.
Mixtures can be classified into two types :
- Homogeneous mixture : The mixture in which all the components are in the same stage and phase are known as homogeneous mixtures. For example : air, solution etc.
- Heterogeneous mixture : The mixture in which all the components are in different stage and phase are known as heterogeneous mixtures. For example : Milk, Cloud, Smoke etc.
Physical and Chemical Changes
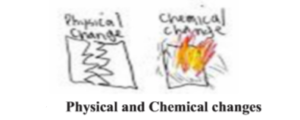
Whenever a matter varies its physical and chemical properties it is known as a change. For example : colour, smell, state, nature, molecular formula etc. Change of any type is a process. These changes can be of two types.
- Physical change : Changes in which the chemical properties of matter remain the same but the physical properties changes, are known as physical change. For example : Water is obtained on heating ice, here the solid state liquefies but chemically both are the same i.e. the molecular formula for both is HO. 2 H2O(s) ® H2O(l) ice – water
- Chemical change : Changes in which the chemical properties of matter changes i.e. a new chemical substance is formed as a result of the change, are known as chemical change. Example : Carbon-di-oxide is obtained on burning carbon in oxygen. Here Carbon is in solid state and oxygen is in gaseous state. The carbon-di-oxide obtained is in gaseous state. Here along with the state even the molecular formula has changed C(s) + O2(g) ® CO2(g) [Carbon + Oxygen = Carbon-di-oxid]
- In the given picture tearing of paper is a physical change while its burning is a chemical change.
State Change of Matter and its Effect
Whenever there is a change in the state of matter, primarily, the distance between its particles, the energy of its particles and the position of the particles change.
- Effect of Temperature :
- On being heated the kinetic energy of the particles increases. When solid is heated, the rate of vibration of its particles increases. The energy provided by the heat extends across the force of attraction between the particles. Because of this the particles leave their set location and start moving independently. A stage is reached when the solid melts and become liquid.
- The temperature at which the solid melts down to liquid is termed as the melting point of that substance. The melting point of ice is 273.16ºK. The process of melting i.e., conversion of solid into liquid state is also known as fusion.
- “The heat energy required to change one kilogram of a solid into liquid, at its melting point, under one atmosphere pressure, is known as the latent heat or enthalpy of fusion i.e. melting.”
- On being heated, the kinetic energy of liquid particles increase further resulting in the conversion to gaseous state. The temperature at which liquid changes to gas is known as its boiling point.
- “The heat energy required to convert one kilogram of liquid, at one atmosphere pressure, at its boiling point, into the vapour state, is known as the latent heat or enthalpy of vapourisation”.
- Effect of Pressure : When pressure is applied the gas particles come close together. When the distance between these particles decreases, gaseous state changes into to the liquid state. But this liquid cannot be solidified by applying immense pressure because the compressibility of liquid is very less.
Purification of Matter
In nature majority of the matter is present in impure form. Hence their purification is essential. There are different methods of purifying different substances.
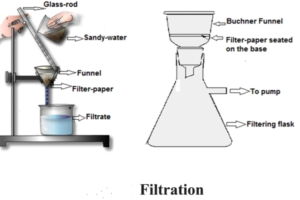
- Filtration : Filtration is a method to separate solid from liquid in a heterogenous mixture. In filtration the solid matter is collected on the filter paper in the form of a residue and the liquid is obtained as filterate, when the mixture is passed through a filter paper. Example : Separating water from the sandy water.
- Crystallisation : Crystallisation is the phenomenon of formation of solid crystals from the saturated solution. The crystallisation technique of separating solid from liquid starts with vapourisation. However, in crystallisation when the solution becomes very concentrated, vapourisation is stopped. The concentrated solution thus obtained when cooled gradually, results in the formation of crystals, which can be seperated by filteration. For example – seperating sugar from sugar syrup, making crystal sugar, obtaining salt crystals from saline solution etc.
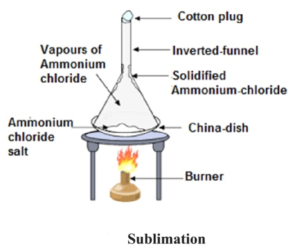
- Sublimation : Sublimation is the property of some substances, by virtue of which, they change directly from solid state to vapour state without liquifying and the vapours when cooled form solid without changing over to the liquid state. For example : Ammonium chloride (Sal ammoniac), iodine, camphor, Naphthalene etc exhibits the property of sublimation. Separating a mixture of salt and sal ammoniac (Sodium chloride and Ammonium chloride) : On being heated, the sal ammoniac from the mixture vapourises while the salt is left behind. These vapours cool down on the inverted funnel placed on the mixture being heated and thus converts back into pure sal ammoniac.
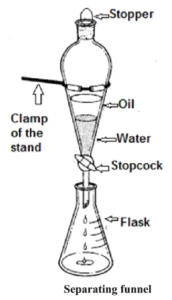
- Differential extraction : This is a technique to separate two immiscible liquids from each other. For example oil and water. When poured in a separating funnel the two liquids in the mixture form separate layers. When the stop- cork is opened, the heavy liquid comes out first then the lighter liquid is obtained.
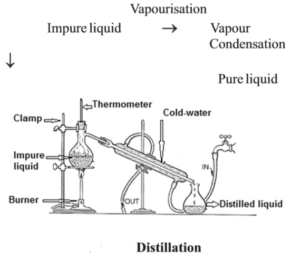
- Distillation : When soluble solid is present in a liquid, the liquid from the mixture vapourises on being heated and on cooling these vapours, pure liquid is obtained because of condensation. This process is known as distillation. Example water distillation etc.
- Fractional Distillation : If the boiling point of two liquids do not have sufficient difference, they cannot be separated by simple distillation. Such liquids vapourise at the same temperature range and then condense together. In such a situation fractional distillation is made use of.

- When the mixture is heated each liquid vapourises at its boiling point. Their vapours when passed through fractioning column and condensed, yield the different liquids. First the liquid with lower boiling point is obtained and then, that with higher boiling point. Example : Various components like petrol, diesel, kerosene, vaseline etc are separated from petroleum by fractional distillation.
PRACTICE QUESTION PAPER
Objective type questions :
Q. 1 When did Maharshi Kanad provided information regarding atom?
(a) 500 BC (b) 100 BC (c) 460 BC (d) 1808 AD
Q. 2 The atomicity of oxygen in ozone is :
(a) 1 (b) 2 (c) 3 (d) 4
Q. 3 Which of the following substance is not solid at room temperature ?
(a) Salt (b) Alum (c) Oxygen (d) Sal ammoniac
Q. 4 The temperature at which liquid changes to solid is known as :
(a) melting point (b) boiling point
(c) freezing point (d) condensation temperature
Q. 5 Which of the following options, is a mixture?
(a) Water (b) Brass (c) Iron (d) Salt
Very short answer type questions :
Q. 6 Who was the first person to impart information about atom?
Q. 7 What is the approximate size of atom?
Q. 8 Write the molecular formula of water.
Q. 9 Which type of mixture, is the mixture of oil and water?
Q. 10 By which method can water be separated from the sandy water?
Q. 11 What is the atomicity of oxygen in di- oxygen?
Q. 12 Give an example of a monoatomic molecule.
Q. 13 Which is the state of matter whose shape and volume are constant?
Q. 14 Give the full form of C.N.G.
Q. 15 What is the process of conversion of liquid into vapours, known as?
Short answer type questions :
Q. 16 What is melting point? Define.
Q. 17 Write the definition of the latent heat of vapourisation?
Q. 18 Write one difference between an element and a compound?
Q. 19 What is a mixture? Give one example.
Q. 20 Write four properties of the liquid state.
Q. 21 What is a physical change? Give one example.
Q. 22 Explain the effect of pressure on the liquefaction of gases.
Q. 23 Write any three specialities of matter.
Q. 24 Which type of mixture can be separated using a separating funnel?
Q. 25 Define compound. Give one example.
Essay type Questions :
Q. 26 Explain the effect of temperature on the conversion of different phases of matter.
Q. 27 Explain sublimation with the help of a well labelled diagram.
Q. 28 Write three differences between solid, liquid and gas.
Q. 29 Write two differences between physical and chemical changes.
Q. 30 How is matter purified by distillation? Explain with the help of a diagram.
Recent Post :
- Environmental Pollution : 5 Types, Effect & Causes of Pollutions and How to control on it
- Celestial bodies and Indian Calendar
- OPPO Unveils Reno8 Pro House of the Dragon Limited Edition : Book Here at lowest price, Don’t Miss it
- Yoga Sutras of Patanjali : Chapter 4: Liberation (Kaivalya Pada)
- Realme Narzo 50 5G : Buy at lowest price @ 12,999/-
- Yoga Sutras of Patanjali : Chapter 3 : Progressing (Vibhuti Pada)
- Yoga Sutras of Patanjali : Chapter 2: Practices (Sadhana Pada)
- LAVA Braze 5G : Cheapest mobile take it home. Don’t miss it
- Yoga Sutras of Patanjali : Chapter-1 : Concentration (Samadhi Pada)
- Ashtang Yog : Do you know it’s 17 Effects on Health
- Surya Namaskar : 12 Steps with pose & it’s benifits
- OPPO A98 : Full Specifications & Price in India
- Concept of Life
- Xiaomi Redmi Note 12 5G : Price, Full Specification & Launch Date
- Structure of Matter and Molecule
- Samsung Galaxy M13 5G buy at lowest price @ Rs. 9,999/-
- 7 Amazing facts about Prime Minister of the United Kingdom Mr. Rishi Sunak
- PM Kisan Tractor Yojana 2022: This Diwali, bring a free tractor at Home
- Social Media : Chapter-1, A complete free course
- Education: New Definition & Meaning and 05 Concepts
Our Partners

