Ecosystem and Biogeochemical cycle
Ecosystem
It is the structural and functional unit of biosphere and is characterized by self sustenance. i.e. ability to continue a healthy state without outside assistance. It is an open system and depends on sun-energy. Ecosystems may be small or big. There is a continuous exchange of minerals and energy between neighbouring ecosystems. Hence, all the ecosystems are interlinked and inter related. The web of inter-linked ecosystems is known as the biosphere. The term ‘ecosystem’ was first of all used by a British Ecologist Arthur Tansley in 1935. It is made up of biotic and abiotic components. According to Eugene P. Odum (1963) ecosystem is the basic unit of ecology in which the biotic and abiotic components interact with each other and both components are important for the continuum (uninterrupted existence) of life.
Animals are holozoic and do not prepare their own food; they depend – directly or indirectly – upon plants for their food requirements. Although plants synthesize their own food yet they depend on various abiotic factors. From a broader perspective, the earth we live upon is itself a giant ecosystem whose various biotic and abiotic components interact with each other. It is because of this reason that structural and functional changes occur continuously in the ecosystem. Although it appears to be impossible to control the entire Bioshpere but to facilitate its study it can be subdivided into various ecosystems.
Structure of Ecosystem
Ecosystem is made up of two main components biotic and abiotic.
- (1) Abiotic components : It includes inorganic, organic and climatic factors like – air, water, soil, sun light etc.
- (i) Inorganic substances : It includes nutritive elements and components like – carbon, nitrogen, sulphur, phosphorus, carbon-di-oxide, water etc. They are cycled in the ecosystem.
- (ii) Organic compounds : It includes proteins, fats, carbohydrates, humic substances etc. They are basically related to the living body and connect the abiotic and biotic components.
- (iii) Climatic factors : They are of two types –
- (a) Environmental factors like sun-light, temperature, humidity, precipitation etc.
- (b) Edaphic factors like topography, soil texture etc.
- (2) Biotic components : The living components of the environment are known as the biotic components. They can be further categorized as producers, consumers and decomposers.
- (i) Producers : These are the chlorophyll containing plants which include algae, grass, trees etc. They convert solar energy into chemical energy during photosynthesis. They are the source of food for majority of animals. They are also termed as autotrophs as they synthesize their own food.
- (ii) Consumers : These are the organisms which cannot synthesize their own food and depend on other organisms for their nourishment. They are known as the consumers and are heterotrophs. Mostly they are animals. Animals which directly depend upon plants for their food are known as herbivorous. For example – grass hopper, goat, sheep, rabbit etc. The animals which depend upon herbivorous animals for their food requirement are known as carnivorous. For example snake, lion, frog etc. They may be predators or parasites. The animals which can derive their food from both plants and animals are known as omnivorous. For example – cockroach, man etc.
- (iii) Decomposers : This category mainly includes bacteria and fungi. In an ecosystem bacteria generally work upon the animal tissue while fungi on plant tissue. They digest dead organic matter with the help of enzymes and in this way the basic elements of the cell components are released into the atmosphere, which are then reused by producers.
Types of ecosystems
Ecosystem are of two types :
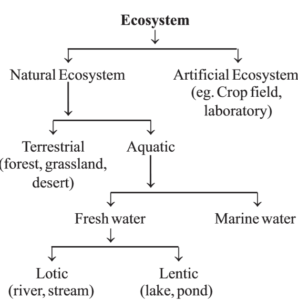
- Natural ecosystem : They are naturally under their own control and are self sustained with the human interference of the least order.
- (a) Terrestrial ecosystem : Example- Forest, grassland, desert etc.
- (b) Aquatic ecosystem : They are of two types
- (i) Fresh water and
- (ii) marine water.
The fresh water ecosystem again may be of two types lotic (example – river, streams etc.) and lentic (example – pond, lake etc.).
- Artificial ecosystem : These ecosystems are man-made and are under their control. Example crop-land which includes fields of wheat, bajra, rice etc. Here man tries to control the biotic community and physico- chemical factors.
Apart from the above systems, even the space eco-system has been recognized.
Biogeochemical cycle
The harmony between the biotic and abiotic components of the biosphere keeps it dynamic and stable. There is transfer of substance and energy between the various components of biosphere due to this co-ordination. Let us consider the various processes that keep this balance.
Water-cycle
You have seen how it rains following vapourisation of water from water bodies and then there condensation. But we have never seen the seas and oceans drying. Then how is water replenished in these reservoirs.
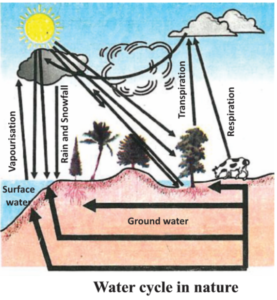
The entire process by which water forms water vapour, forms clouds and then comes back to the earth surface in the form of rains and then flows into the oceans is known as the water cycle.
This cycle is not so easy and simple as it appears to be by the statement given. All the water that comes down to earth does not directly flow into the oceans. Some of it percolates into the soil and forms part of the water table. Some of this ground water flows out in the form of streams on the earth surface or we bring it to the surface through wells and tube wells. For the various life processes the terrestrial living beings use this surface water.
As is a well known fact, water dissolves many substances. When water flows through soluble minerals some of them dissolve in it. These are then carried over to oceans by water flowing in the rivers and rivulets and they are then used by the aquatic flora and fauna.
Oxygen cycle
Oxygen is one of the most abundant element on earth. Its quantity is nearly 21% of the atmospheric gases. It is also present on a large scale, on the earth surface, in the form of water and other compounds and in air in the form of carbon-di-oxide also. It is present in the form of metal and silicon oxides in the earth’s crust. It is also present in the form of carbonate, sulphate, nitrate and oxides of minerals. It is an essential component of biomolecules like – carbohydrates, proteins, nucleic acid and fats or lipids.
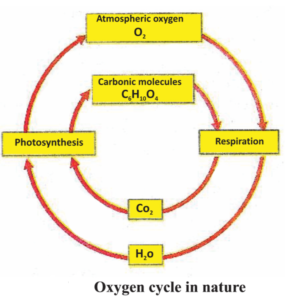
But when we talk about oxygen cycle, we are basically concerned with cycle that keeps the oxygen levels in nature balanced. Oxygen from atmosphere is used in three processes – Respiration, combustion and formation of oxides of nitrogen. Oxygen returns to the atmosphere by only one process i.e. photosynthesis. This forms the outline of oxygen cycle in nature.
Although we consider oxygen to be of importance in the respiratory process but for some organisms, mainly bacteria elemental oxygen is toxic. Actually nitrogen fixation does not take place in the presence of oxygen.
Carbon cycle
Carbon is present on earth in various states. It is present in the form of diamond and graphite in its original form. In atmosphere it is present in the form of a compound – carbon-di-oxide; carbonates of various minerals and hydrogen carbonate. Furthermore, all the life forms are based on carbon- based molecules like – proteins, carbohydrates, fat, nucleic acid and vitamins. The exo and endo skeleton of many organisms is made up of carbonate salts. Carbon is incorporated in various forms of life through the process of photosynthesis which takes place in the presence of sunlight in the chlorophyll containing plants. The carbon-di-oxide present in the atmosphere or in dissolved form in water is converted to glucose by the process of photosynthesis. These molecules of glucose either convert into other molecules or provide energy for the synthesis of other important molecules.
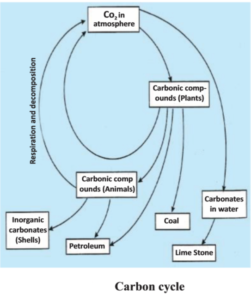
Glucose is used in the process that provides energy to living beings. Glucose is oxidized to carbon-di-oxide, with or without oxygen, by the process of respiration. The carbon-di-oxide thus formed goes back into the atmosphere. Carbon-di- oxide also enters the atmosphere through the process of combustion where fuel is used to cook food, warm it, or in transportation and various industries. Actually after industrial revolution man started using fossil fuels on a large scale, to the extent that the amount of carbon-di-oxide in atmosphere has nearly doubled. Like water even carbon is recycled by various physical and chemical processes.
Nitrogen cycle
Nearly 78% of our atmosphere is made up of nitrogen gas. This gas is the component of many molecules essential for life. For example : proteins, nucleic acid – DNA and RNA and some vitamins.
Nitrogen is found in other bio molecules also like alkaloids and urea. Thus nitrogen is an essential nutrient for all organisms. The life would be simple if all the organisms use nitrogen present in the atmosphere, directly. But this does not happen in nature. Apart from some bacteria, most of the other organisms are unable to convert the inactive nitrogen into nitrates, nitrites etc. “Nitrogen fixing” bacteria are found either as free living forms or in symbiotic association with some types of dicots. Generally, these nitrogen fixing bacteria are present in special root nodules of pod bearing plants. Apart from these bacteria, the nitrogen atoms also form nitrates and nitrites by various physical reactions. The high temperature and pressure generated in the atmosphere at the time of lightening, converts nitrogen to oxides of nitrogen. These oxides dissolve in rain water and form acid which fall on earth surface; then after it is used by various life forms.
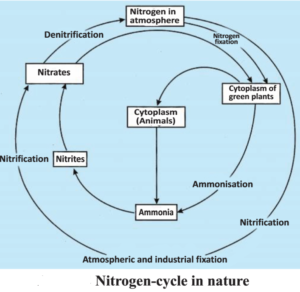
What happens to nitrogen after it forms various nitrogenous molecules. The plants, generally, absorb nitrates and nitrites and convert them into amino-acids which are used in protein synthesis. There are some other bio-chemical options which are used for synthesis of other nitrogen containing complex molecules. These proteins and other complex compounds are then used by the animals. When the plant or animal die, various bacteria present in the soil converts the nitrogenous compounds into nitrates and nitrites and other type of bacteria break down these nitrate and nitrite molecule into nitrogen element.
Thus in nature there operates a nitrogen cycle in which nitrogen, passing from its basic form in atmosphere converts into simple molecules in the soil and water and then in living beings it forms very complex compounds. Later-on they break down, releasing the nitrogen atoms back into the nature.
Green House Effect
In a glass house, the internal temperature is very high as compared to the external temperature because the glass does not allow the heat waves to transmit back in the atmosphere. This concept is made use of in maintaining, warm tropical plants in the cold climate. This type of cover is known as a green house.
Similar, phenomenon occurs in the atmosphere too. Some gases prevent the escape of heat into the outer atmosphere. Increase in the amount of such gases in the atmosphere may rise the average temperature of the earth’s atmosphere. This type of effect is known as the green house effect. Carbon-di-oxide is a green house gas. Increase in the amount of carbon-di-oxide in the atmosphere will increase the heat content of the atmosphere. These activities lead to global warming.
Ozone layer
The elemental oxygen is generaly present in the form of bi-atomic molecule. However, in the upper layer of atmosphere tri-atomic molecules are also present. Its formula is O3 and is known as ozone. In contrast to the biatomic oxygen molecules, the ozone is toxic. We are lucky that ozone is unable to remain near the surface layers of earth. It absorbs the harmful radiations of the sun. Thus it prevents those radiations from reaching the earth surface which may harm various life forms.
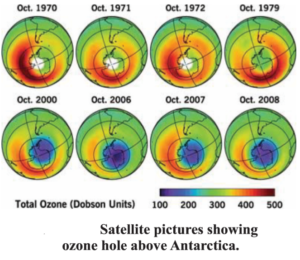
Recently, it has been detected that the ozone layer present in upper atmosphere is degrading. Different types of compounds, made by man, for example the chloro-fluoro-carbons (CFCs) are present in the atmosphere in a stable state. CFCs are chlorine and fluorine containing organic molecules. They are very stable and do not degrade even by biological processes. Once they reach near the ozone molecules, they react with them. This results in decrease in the amount of ozone and this leads to thinning of the ozone layer. Recently ozone hole has been observed above Antarctica. It is very difficult even to think about the hazardous effects the life on earth will be subjected to, in the absence of this ozone layer, which is thinning rapidly. Serious efforts are required to be undertaken to protect it.
Recent Post
-
Environmental Pollution : 5 Types, Effect & Causes of Pollutions and How to control on it
- Celestial bodies and Indian Calendar
- OPPO Unveils Reno8 Pro House of the Dragon Limited Edition : Book Here at lowest price, Don’t Miss it
- Yoga Sutras of Patanjali : Chapter 4: Liberation (Kaivalya Pada)
- Realme Narzo 50 5G : Buy at lowest price @ 12,999/-
- Yoga Sutras of Patanjali : Chapter 3 : Progressing (Vibhuti Pada)
- Yoga Sutras of Patanjali : Chapter 2: Practices (Sadhana Pada)
- LAVA Braze 5G : Cheapest mobile take it home. Don’t miss it
- Yoga Sutras of Patanjali : Chapter-1 : Concentration (Samadhi Pada)
- Ashtang Yog : Do you know it’s 17 Effects on Health
- Surya Namaskar : 12 Steps with pose & it’s benifits
- OPPO A98 : Full Specifications & Price in India
- Concept of Life
- Xiaomi Redmi Note 12 5G : Price, Full Specification & Launch Date
- Structure of Matter and Molecule
- Samsung Galaxy M13 5G buy at lowest price @ Rs. 9,999/-
- 7 Amazing facts about Prime Minister of the United Kingdom Mr. Rishi Sunak
- PM Kisan Tractor Yojana 2022: This Diwali, bring a free tractor at Home
- Social Media : Chapter-1, A complete free course
- Education: New Definition & Meaning and 05 Concepts
Our Partners

